Note
This is a cross-post from John Stowers' personal blog where this article was originally published.
I'm proud to announce the availability of all source code,
and the advanced online publication
of our paper
Bath DE*, Stowers JR*, Hörmann D, Poehlmann A, Dickson BJ, Straw AD (* equal contribution) (2014)
FlyMAD: Rapid thermogenetic control of neuronal activity in freely-walking Drosophila.
Nature Methods. doi 10.1038/nmeth.2973
FlyMAD (Fly Mind Altering Device) is a system for targeting freely walking
flies (Drosophila) with lasers. This allows rapid thermo- and opto- genetic manipulation of the
fly nervous system in order to study neuronal function.

The scientific aspects of the publication are better summarised on
nature.com,
here,
on our laboratory website, or
in the video at the bottom of this post.
Briefly however; if one wishes to link function to specific neurons one could conceive of
two broad approaches. First, observe the firing of the neurons in
real time
using fluorescence or other microscopy techniques. Second, use genetic techniques to engineer organisms with
light or temperature sensitive proteins bound to specific neuronal classes such that by the application
of heat or light, activity in those neurons can be modulated.
Our system takes the second approach; our innovation being that by using real time computer vision and
control techniques we are able to track freely walking Drosophila and apply precise (sub 0.2mm)
opto- or thermogenetic stimulation to study the role of specific neurons in a diverse array of behaviours.
This blog post will cover a few of the technical and architectural decisions I made in the creation of the system.
Perhaps it is easiest to start with a screenshot and schematic of the system in operation
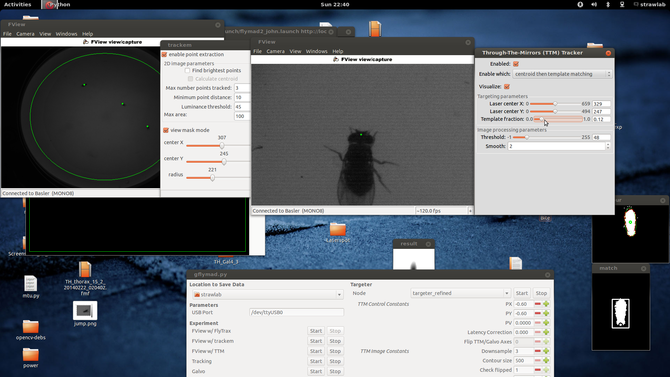
Here one can see two windows showing images from the two tracking cameras, associated image processing configuration parameters
(and their results, at 120fps). In the center at the bottom is visible the ROS based experimental control UI.
Schematically, the two cameras and lasers are arranged like the following
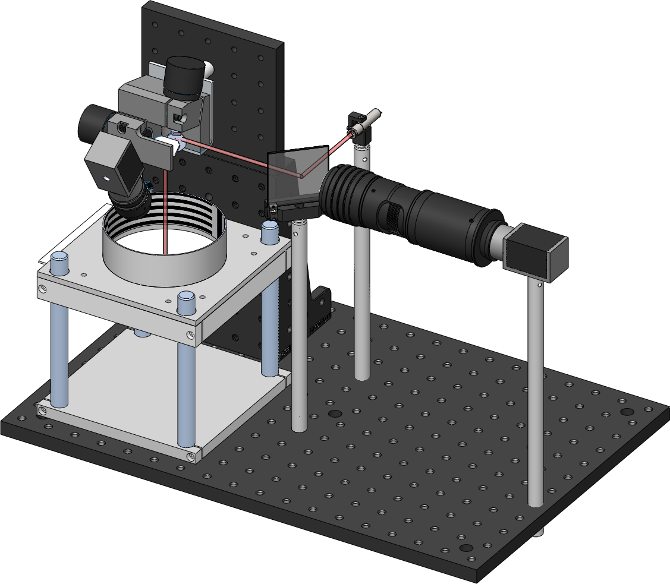
In this image you can also see the thorlabs 2D galvanometers (top left), and the dichroic mirror
which allows aligning the camera and laser on the same optical axis.
By pointing the laser at flies freely walking in the arena below, one can subsequently
deliver heat or light to specific body regions.
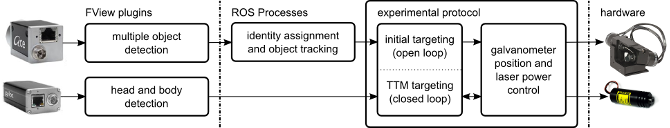
General Architecture
The system consists of hardware and software elements. A small microcontroller and
digital to analogue converter generate analog control signals to point the
2D galvanometers and to control laser power. The device communicates with the host
PC over a serial link. There are two cameras in the system; a wide camera for fly position
tracking, and a second high magnification camera for targeting specific regions of the fly.
This second camera is aligned with the laser beam, and its view can be pointed
anywhere in the arena by the galvanometers.
The software is conceptually three parts; image processing code, tracking and targeting code, and
experimental logic. All software elements communicate using robot operating
system (ROS) interprocess communication layer. The great majority of
code is written in python.
Robot Operating System (ROS)
ROS is a framework traditionally used for building
complex robotic systems. In particular it has a relatively good performance and
simple, strongly typed, inter-process-communication framework and serialization format.
Through its (pure) python interface one can build a complex system of multiple
processes who communicate (primarily) by publishing and subscribing to
message "topics". An example of the ROS processes running during a FlyMAD
experiment can be seen below.
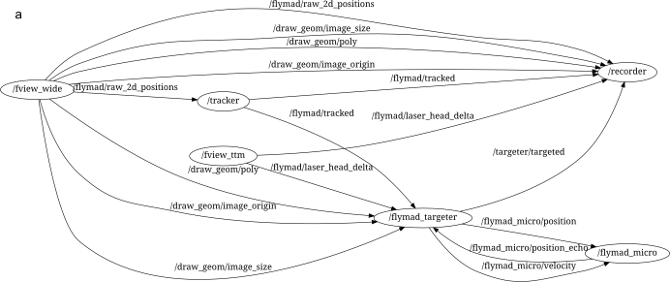
The lines connecting the nodes represent the flow of information across the
network, and all messages can be simultaneously recorded (see /recorder)
for analysis later. Furthermore, the isolation of the individual processes
improves robustness and defers some of the responsibility for realtime
performance from myself / Python, to the Kernel and to my overall
architecture.
For more details on ROS and on why I believe it is a good tool for
creating reliable reproducible science, see my
previous post,
my Scipy2013 video and
presentation
Image Processing
There are two image processing tasks in the system. Both are implemented as
FView
plugins and communicate with the rest of the system using ROS.
Firstly, the position of the fly (flies) in the arena, as seen by the
wide camera, must be determined. Here, a simple threshold approach is used to
find candidate points and image moments around those points are used to find the
center and slope of the fly body. A lookup table is used to point the
galvanometers in an open-loop fashion approximately at the fly.
With the fly now located in the field of view of the high magnification camera a
second real time control loop is initiated. Here, the fly body or head is detected,
and a closed loop PID controller finely adjusts the galvanometer position to achieve
maximum targeting accuracy. The accuracy of this through the mirror (TTM) system asymptotically
approaches 200μm and at 50 msec from onset the accuracy of head detection is 400 ± 200 μm.
From onset of TTM mode, considering other latencies in the system (gigabit ethernet, 5 ms,
USB delay, 4 ms, galvanometer response time, 7 ms, image processing 8ms, and image
acquisition time, 5-13 ms) total 32 ms, this shows the real time
targeting stabilises after 2-3 frames and comfortably operates at better than 120 frames
per second.

To reliably track freely walking flies, the head and body step image processing
operations must take less than 8ms. Somewhat frustratingly, a traditional template
matching strategy worked best. On the binarized, filtered image, the largest contour
is detected (c, red). Using an ellipse fit to the contour points (c,green), the contour
is rotated into an upright orientation (d). A template of the fly (e) is compared with the
fly in both orientations and the best match is taken.
I mention the template strategy as being disappointing only because I spent considerable time
evaluating newer, shinier, feature based approaches and could not achieve the closed loop
performance I needed. While the newer descriptors, BRISK, FREAK, ORB were faster than the previous
class, nether (in total) were significantly more reliable considering changes in illumination than
SURF - which could not meet the <8ms deadline reliably. I also spent considerable time testing
edge based (binary) descriptors such as edgelets, or edge based (gradient) approaches such as
dominant orientation templates or gradient response maps. The most promising of this class was local
shape context descriptors, but I also could not get the runtime below 8ms. Furthermore, one advantage
of the contour based template matching strategy I implemented, was that graceful degradation was
possible - should a template match not be found (which occurred in <1% of frames), an estimate
of the centre of mass of the fly was still present, which still allowed degraded targeting performance.
No such graceful fallback was possible using feature correspondence based strategies.
There are two implementations of the template match operation - GPU and CPU based. The CPU matcher
uses the python OpenCV bindings (and numpy in places), the GPU matcher uses cython to wrap a small
c++ library that does the same thing using OpenCV 2.4 Cuda GPU support (which is not otherwise
accessible from python). Intelligently, the python OpenCV bindings use numpy arrays to store
image data, so passing data from Python to native code is trivial and efficient.
I also gave a presentation
comparing different strategies of interfacing python with native code. The
provided source code
includes examples using python/ctypes/cython/numpy and permutations thereof.
The GPU code-path is only necessary / beneficial for very large templates and
higher resolution cameras (as used by our collaborator) and in general the CPU
implementation is used.
Experimental Control GUI
To make FlyMAD easier to manage and use for biologists I wrote a small GUI using
Gtk (PyGObject), and my ROS utility GUI library
rosgobject.

On the left you can see buttons for launching individual ROS nodes. On the right
are widgets for adjusting the image processing and control parameters (these
widgets display and set ROS parameters). At the bottom are realtime statistics showing
the TTM image processing performance (as published to ROS topics).
Like good ROS practice, once reliable values are found for all adjustable parameters
they can be recorded in a roslaunch file allowing the whole system to
be started with known configuration from a single command.
Manual Scoring of Videos
For certain experiments (such as courtship) videos recorded during the experiment
must be watched and behaviours must be manually annotated. To my surprise, no tools
exist to make this relatively common behavioural neuroscience task any easier
(and easier matters; it is not uncommon to score 10s to 100s of hours of videos).
During every experiment, RAW uncompressed videos
from both cameras are written to disk (uncompressed videos are chosen for performance reasons, because
SSDs are cheap, and because each frame can be precisely timestamped).
Additionally, rosbag files record the complete state of the experiment at
every instant in time (as described by all messages passing
between ROS nodes). After each experiment finishes, the uncompressed videos from
each camera are composited together, along with metadata such as the frame
timestamp, and a h264 encoded mp4 video is created for scoring.
After completing a full day of experiments one can then score / annotate
videos in bulk. The scorer is written in Python, uses Gtk+ and PyGObject for the
UI, and vlc.py for decoding the
video (I chose vlc due to the lack of working gstreamer PyGObject support on Ubuntu
12.04)
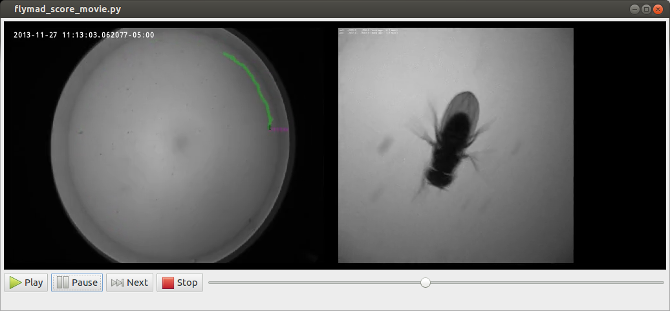
In addition to allowing play, pause and single frame scrubbing through the video,
pressing any of qw,as,zx,cv pairs of keys indicates that a
a behaviour has started or finished. At this instant the current video frame is
extracted from the video, and optical-character-recognition is performed on the
top left region of the frame in order to extract the timestamp. When the video
is finished, a pandas dataframe is created which contains
all original experimental rosbag data, and the manually annotated behaviour
against on a common timebase.
Distributing complex experimental software
The system was not only run by myself, but by collaborators,
and we hope in future, by others too. To make this possible we generate a single
file self installing executable using makeself,
and we only officially support one distribution - Ubuntu 12.04 LTS and x86_64.
The makeself installer performs the following steps
- Adds our Debian repository to the system
- Adds the official ROS Debian repository to the system
- Adds our custom ROS stacks (FlyMAD from tarball and rosgobject from git)
to the ROS environment
- Calls rosmake flymad to install all system dependencies and build
and non-binary ROS packages.
- Creates a FlyMAD desktop file to start the software easily
We also include a version check utility in the FlyMAD GUI which notifies the user
when a newer version of the software is available.
The Results
Using FlyMAD and the architecture I have described above we created a novel system
to perform temporally and spatially precise opto and thermogenetic activation
of freely moving drosophila. To validate the system we showed distinct timing
relationships for two neuronal cell types previously linked to courtship song, and
demonstrated compatibility of the system to visual behaviour experiments.
Practically we were able to develop and simultaneously operate this complex
real-time assay in two countries. The system was conceived and built in approximately
one year using Python. FlyMAD utilises many best-in-class libraries and frameworks
in order to meet the demanding real time requirements (OpenCV, numpy, ROS).
We are proud to make the entire system available to the Drosophila community
under an open source license, and we look forward to its adoption by our peers.
For those still reading, I encourage you to view the supplementary video below,
where its operation can be seen.
Comments, suggestions or corrections can be emailed to me
or left on Google Plus